New International Consensus on CGM & AID Use in Pregnant Women with Diabetes
Dec 23, 2025
Last week, the long-awaited International Consensus Statement on the use of CGM and AID systems in pregnancy was published in The Lancet Diabetes & Endocrinology.
The consensus provides an updated overview of the literature and concrete recommendations on how to use continuous glucose monitoring (CGM) and automated insulin delivery (AID) systems:
- before pregnancy
- during pregnancy
- during labour and delivery
- and postpartum
Importantly, recommendations are clearly differentiated for:
- Type 1 diabetes
- Type 2 diabetes
- Gestational diabetes
Get Access To Updated Diabetes Technology Courses

The statement was initiated by the diaTribe Foundation and endorsed by 24 international societies, including European Association for the Study of Diabetes, International Diabetes Federation and American Association of Clinical Endocrinology. (Notably, it was not endorsed by the ADA.)
Below, we summarise the key practical takeaways most relevant for endocrinologists and diabetes educators.
As a reminder
- TIR = Time In Range 70-180 mg/dl (3.9-10 mmol/l)
- TIRp = pregnancy specific TIR 63-140 mg/dl (3.5-7.8 mmol/l)
CGM and AID use in women with TYPE 1 diabetes
Pre-pregnancy
- HbA1c target:
- <7.0% (53 mmol/mol), or <6.5% (48 mmol/mol) if feasible
- CGM: recommended for all women with type 1 diabetes
- Target TIR >70%
- Preferably target TIRp >70%
- AID:
- Systems with RCT (randomised controlled trial) evidence and showing clinically relevant benefits are recommended already pre-conception.
- Alternatively, AID systems with RCT evidence without clinically relevant benefits might be considered.
During pregnancy
- HbA1c targets:
- 1st trimester: <6.5% (48 mmol/mol)
- 2nd & 3rd trimester: <6.0% (42 mmol/mol)
- Mean sensor glucose target:
- 108–120 mg/dL (6.0–6.7 mmol/L)
- CGM: recommended for all women with type 1 diabetes
- Target TIRp >70%
- Target TBRp <63 mg/dL (<3.5 mmol/l) <4%
- Target TBR <54 mg/dL (<3.0 mmol/l) <1%
- AID:
- Systems with RCT evidence and showing clinically relevant benefits are recommended for all women with type 1 diabetes.
- Alternatively, AID systems with RCT evidence without clinically relevant benefits might be considered when used with assistive techniques by experienced teams.
Intrapartum & postpartum
- CGM: recommended for all women with type 1 diabetes
- Intrapartum target TIRp >70%
- Postpartum target TIR >70%
- AID: continuing AID systems during labour and postpartum:
- maintains tight glycaemic control without increased risk of hypoglycemia
- avoids unnecessary switching to IV insulin
- is safe in early and late postpartum
CGM use in pregnant women with TYPE 2 diabetes
Pre-pregnancy
- HbA1c target: <6.5% (48 mmol/mol), or <6.0% (48 mmol/mol) if feasible
- CGM: may help reach glycaemic goals pre-conception
During pregnancy
- CGM: can be offered based on resources and individual preference
- Suggested CGM target:
- Target TIRp >80%
- Target TBRp <4%
- However, the most appropriate targets are unknown
Postpartum
- When CGM is used:
- Target TIR >70%
CGM use in pregnant women with GESTATIONAL diabetes
- CGM: can be offered based on availability and individual preference
- Suggested CGM target:
- Target TIRp >90%
- However, the most appropriate targets are unknown
This is the first time such a target has been proposed for gestational diabetes—and it sends a clear signal about how tight control should be when CGM is available.
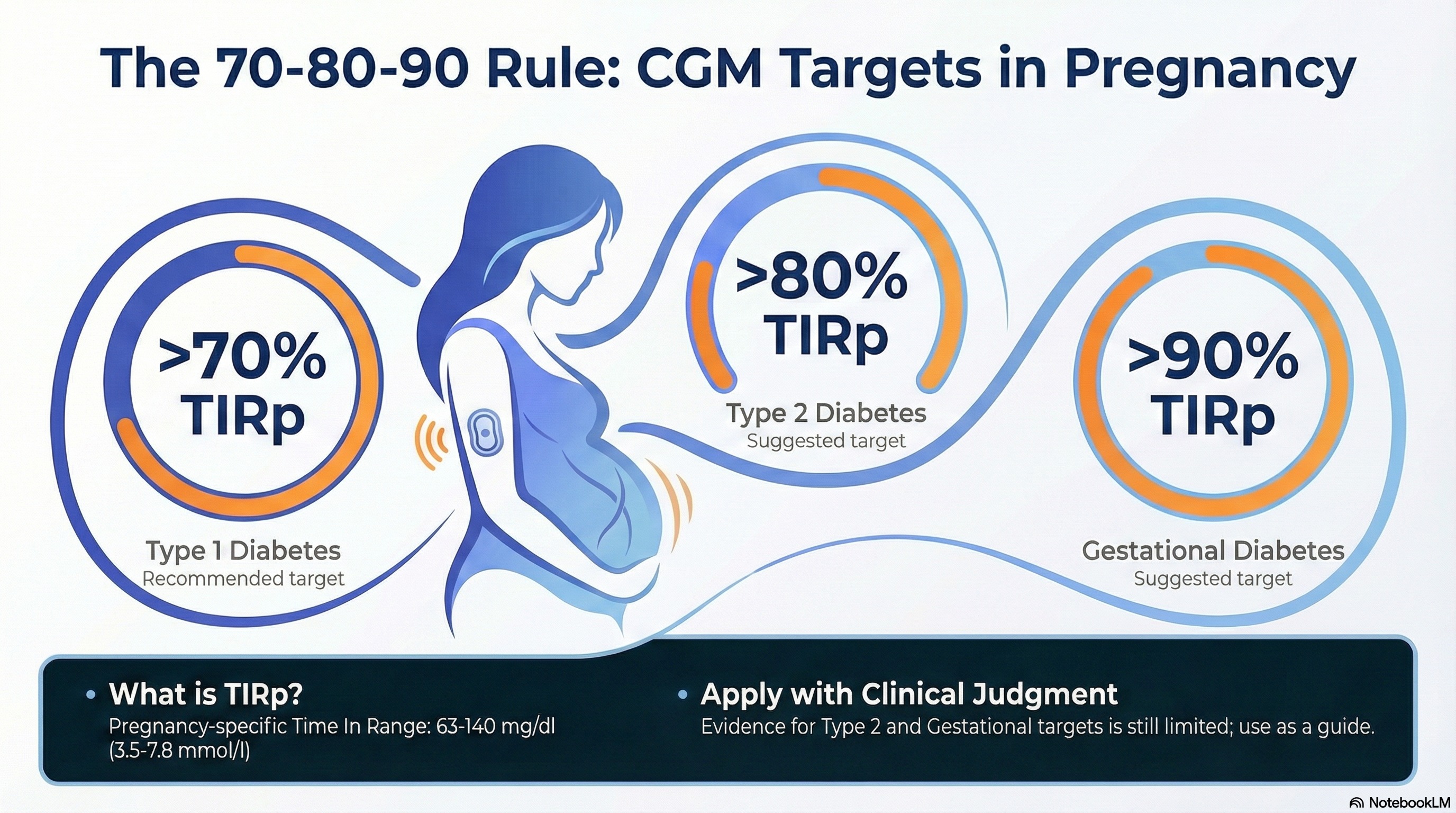
A 70–80–90 rule
Infographic made with NotebookLM based on this blog section
Simple. Memorable. Actionable.
- CGM target for Type 1 diabetes: TIRp >70%
- CGM target for Type 2 diabetes: TIRp >80%
- CGM target for Gestational diabetes: TIRp >90%
This 70–80–90 rule brings clarity and can give a practical mental model to guide care.
Important caveat: the consensus also stresses that robust evidence for CGM targets in pregnant women with type 2 diabetes and gestational diabetes is still limited. These thresholds should therefore be applied with clinical judgment, not as rigid cut-offs.
AID in pregnancy: system-specific guidance
Another strong contribution of this consensus is its system-specific, real-world guidance on how to use AID systems during:
- pregnancy
- labour
- postpartum
Including when and how to use:
- lower glucose targets
- strengthened carb ratios
- assistive techniques (yes, including fake carbs)
- temporary overrides
- postpartum profiles
This is not theoretical guidance—it reflects what experienced teams are already doing successfully.
#1 MiniMed 780G
- Pregnancy:
- Lowest glucose target: 100 mg/dL (5.5 mmol/L)
- Active insulin time: 2 hours
- Strengthen carb ratios; if safe meal bolus occurs when strengthening meal carb ratios, relax carb ratios and add extra fake carbs with meals
- Bolus 10-15 minutes before meals
- Intrapartum:
- Target usually unchanged (100 mg/dL | 5.5 mmol/L), increase to 110 or 120 mg/dl | 6.1-6.7 mmol/l) if needed
- Postpartum:
- Target usually unchanged (100 mg/dL | 5.5 mmol/L), increase to 110 or 120 mg/dl | 6.1-6.7 mmol/l) if needed, temporary target (150 mg/dl | 8.3 mmol/l) rarely needed
- Active insulin time: 2 hours
- Reduce carb ratio by at least 50% (80% when breastfeeding)
#2 Tandem Control-IQ
- Pregnancy:
- Use Sleep mode 24/7
- Do not accept bolus reductions when glucose <110 mg/dL (6.1 mmol/L)
- Consider super-bolus strategies
- Need to split boluses if > 25 units or use U200 off-label
- Strengthen carb ratios, basal settings, and sensitivity
- Bolus 10–15 min before meals and later in pregnancy as needed up to 30–45 min before meals
- Intrapartum:
- Continue Sleep activity
- Postpartum:
- Activate postpartum profile* immediately
- Sleep activity can often be continued
- If (fear of) hypoglycaemia: switch to standard Control-IQ or temporarily use Exercise activity
#3 CamAPS FX (mylife Loop)
- Pregnancy:
- Glucose target 100 mg/dL (5.5 mmol/L) before 16 weeks
- Glucose target 90 mg/dL (5.0 mmol/L) from 16 weeks
- Overnight glucose targets as low as 80 mg/dL (4.5 mmol/L)
- Use boost as needed for 2-4 h after large meals (from 20 weeks)
- Update weight every trimester
- Bolus 10–15 min before meals
- Intrapartum:
- Increase target to 100–110 mg/dL (5.5–6.0 mmol/L)
- Carb ratios of between 1:12g and 1:15g
- Postpartum:
- Glucose target ~110 mg/dL (6.0 mmol/L)
- Use Ease-Off as needed
- Carb ratios of between 1:12g (non-breastfeeding) and 1:15g (breastfeeding)
#4 Omnipod 5
- Pregnancy:
- Lowest glucose target 110 mg/dL (6.1 mmol/L)
- Lower correction factors in 2nd and 3rd trimester
- Short insulin action (2–3 hours)
- Expect frequent additional manual corrections, especially post-prandial
- Reverse correction off
- If glucose <110 mg/dL (6.1 mmol/L) and trending down: manual glucose entry does not reduce bolus
- Exit AID overnight as needed
- Intrapartum:
- No evidence-based recommendation
- Postpartum:
- Glucose targets 110–150 mg/dL (6.1-8.3 mmol/L)
- Consider resetting the pump to prepregnancy settings
- Temporary use of manual mode may be helpful before resuming automation
#5 Twiist Loop & open-source AID (Loop, AndroidAPS, iAPS, Trio)
- During pregnancy:
- Daytime target 90-100 mg/dl (5.0-5.5 mmol/L) before 16 weeks
- Consider night-time target ≤ 90 mg/dl (5.0 mmol/L) if variability is low
- Consider lower targets after 16 weeks
- Liberal use of post-meal high custom overrides
- Consider pre-meal target versus pre-meal bolusing
- Twiist Loop & DIY Loop: consider LolliPop (30 min) food absorption after 16 weeks
- Intrapartum:
- Prepare 2 temporary overrides in advance (50 and 75% insulin delivery, with a target of 100 mg/dl (5.5 mmol/L, but activate only when clinically needed
- Postpartum:
- Increase glucose targets to 110–135 mg/dL (6.1–7.5 mmol/L)
- Continue temporary overrides until postpartum settings* are active
- AndroidAPS: if experience is limited: stop dynamic ISF, reduce max basal, max IOB, and max bolus by 25–50%
#6 iLet Bionic Pancreas
- During pregnancy:
- Lowest glucose target of 110 mg/dl (6.1 mmol/L)
- Update weight for gestational weight gain
- Intrapartum:
- No recommendation due to absence of evidence
- Postpartum:
- Adapt glucose targets between 110-130 mg/dl (6.1-7.2 mmol/L) as needed
#7 Diabeloop
- During pregnancy:
- Lowest glucose target 100 mg/dl (5.5 mmol/L)
- Increase aggressiveness in hyperglycaemia, normoglycaemia, and at meals
- Intrapartum:
- No recommendation due to absence of evidence
- Postpartum:
- Adapt glucose targets between 100-130 mg/dl (5.5-7·2 mmol/L)
- Adapt aggressiveness in hyperglycaemia, normoglycaemia, and at meals
Postpartum profile – general principles
For AID systems with setting-based algorithms (e.g. Control-IQ and open-source systems), basal rates, carbohydrate-to-insulin ratios, and insulin sensitivity factors must be weakened immediately after delivery—or just before delivery if more practical—by switching to an individualised postpartum profile.
How to define the postpartum profile:
- If pre-pregnancy pump settings were optimised and are available: reduce all settings by at least 20% compared with pre-pregnancy values - apply larger reductions when breastfeeding
- If pre-pregnancy settings are unavailable or unreliable: use settings that are at least 50% less aggressive than late-pregnancy settings
Implementation:
- Systems that support multiple profiles (e.g. Control-IQ, AndroidAPS): programme a dedicated postpartum profile in advance and activate immediately after delivery
- Systems without multiple profiles (e.g. Twiist Loop, DIY Loop): postpartum settings must be programmed manually after delivery, including basal rates, carb ratios, correction factors and glucose targets.
Key point: postpartum insulin needs change abruptly. Preparation prevents hypoglycaemia.
Final thoughts
Many questions remain unanswered.
But this consensus marks a few steps forward.
For the first time, clear TIR targets for pregnancy in type 2 diabetes and gestational diabetes are proposed—and backed by a broad, international expert group.
Where hard RCT data still lags behind, these recommendations offer the best possible guidance for caring for pregnant women today, using the technology we already have.
We are grateful to the writing group for bringing structure and clarity to a complex area of care—and we look forward to the next wave of evidence that will refine these recommendations even further.
Step by step, diabetes technology is making pregnancy safer, more predictable, and more humane.
Kind regards,