AID Systems Update Spring 2024
Mar 21, 2024
Now that the Advanced Technologies & Treatments for Diabetes (ATTD) conference has concluded and companies have disclosed their quarterly reports, there's a wealth of new information to share regarding diabetes technology.
Given the breadth of updates, attempting to condense them into just 10 points would be inadequate.
Therefore, I've opted to provide a comprehensive market overview detailing the latest developments for each device.
Get Access To Updated Diabetes Technology Courses
In this blog post, you'll receive an in-depth update on the current landscape of Automated Insulin Delivery (AID) systems.
We'll delve into:
- Current usage statistics,
- Real-world performance outcomes,
- Timelines for anticipated product enhancements,
- Expansions in indications (including applications in pregnancy, preschoolers, type 2 diabetes, and other insulin variations).
Additionally, we'll explore advancements towards achieving fully integrated AID systems, such as improved meal management capabilities.
Continue reading to stay informed about the latest advancements in the realm of the "artificial pancreas"!
1. MiniMed 780G
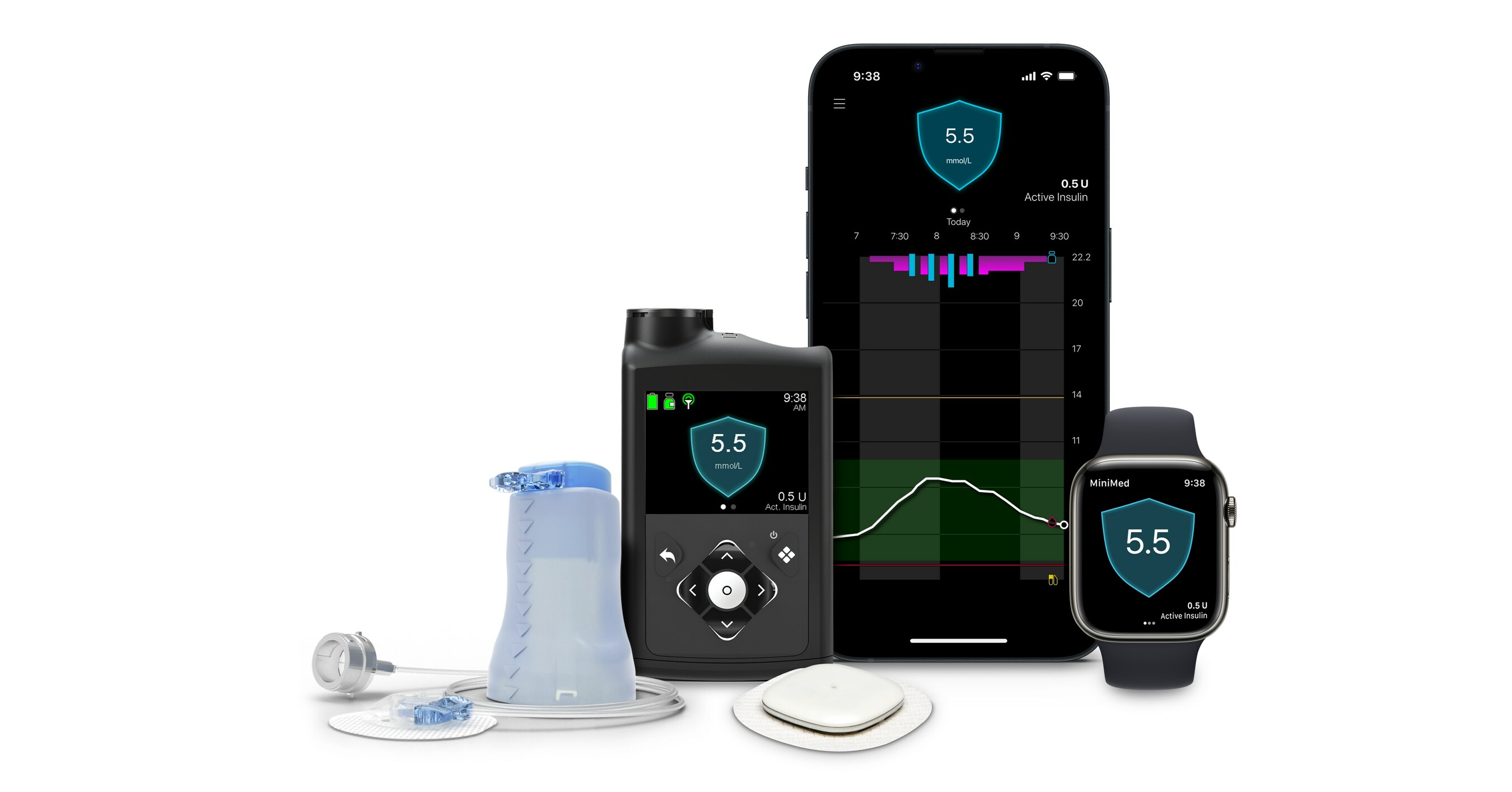
MiniMed 780G Usage and Real-World Performance
- The precise number of MiniMed 780G users has not been disclosed by the company over the past four years. However, based on quarterly updates, it's estimated that approximately 600,000 individuals worldwide are utilizing this system, making it the most widely used AID system.
- Recent real-world data, including a study of over 100,000 individuals, demonstrated a mean Time In Range (TIR) of 72.3% and Time Below Range (TBR) of 2.0%. Furthermore, findings presented at ATTD2024 showcased promising results for initial users in the United States, with a mean TIR of 78% and TBR of 1.5% among 7,499 individuals using the MiniMed 780G with Guardian 4 sensors.
Updates to the MiniMed 780G Product
- A significant update on the horizon is the transition from the Guardian 4 sensor to the Simplera Sync sensor. The Simplera Sync sensor received CE-label approval in January 2024 and is slated for commercialization outside the US this summer, with an FDA-label submission expected in the first half of 2024. While a pivotal study utilizing this new sensor has been completed, the results have not yet been disclosed.
- Medtronic has hinted at the development of an 800-series pump and patch pump, as well as advancements in algorithmic capabilities and automatic meal handling, though precise timelines for these updates remain unclear.
MiniMed 780G in Special Populations
- Results from the CRISTAL study, presented by Prof. Benhalima at ATTD2024, highlighted the MiniMed 780G's performance during pregnancy. While it did not result in a reduction of Time In Range during pregnancy (TIRp), it did show improvements in TIRp overnight, reduced TBRp, decreased hypoglycemia unawareness, reduced glycemic variability, and enhanced treatment satisfaction.
-
For preschoolers aged 2-6 years, the approval status of MiniMed 780G remains pending. Encouraging findings from a small-scale study conducted in Finland in February 2023 have demonstrated favorable outcomes. Medtronic intends to leverage the Lenny trial to pursue FDA approval for this age group.
-
In the type 2 diabetes management, significant strides have been made with the MiniMed 780G. Real-world data unveiled at ATTD2024 showcased the experiences of 617 individuals self-identified as having type 2 diabetes. Impressively, they achieved a Time In Range (TIR) of 75% and Time Below Range (TBR) of 1.1%. These outcomes closely mirror the performance observed in individuals with type 1 diabetes. Moreover, an ongoing pivotal trial in the United States involving over 500 participants with type 2 diabetes promises to further elucidate the device's efficacy in this population.
MiniMed 780G Compatibility with Newer Insulins
- The MiniMed 780G is currently approved for use with Novorapid, Novolog, or Humalog 100 U/ml insulins.
- Recent updates indicate that it has also secured CE-label approval for use with Fiasp and Lyumjev. Studies evaluating Fiasp in individuals with type 1 diabetes and the MiniMed 780G are underway, while preliminary findings with Lyumjev showcased comparable overall performance to Humalog, with slightly improved postprandial glucose excursion and lower TBR.
Advancements Towards a Full AID System
- Recent research has demonstrated that the MiniMed 780G functions effectively with simplified meal announcements. Additionally, a smartwatch-based meal detection algorithm developed by Medtronic has shown promise, as presented at ADA2022. These developments signal progress towards achieving a fully integrated AID system.
2. Tandem Control-IQ technology

Tandem Control-IQ Usage and Real-World Performance
- Estimates suggest that approximately 300,000 individuals worldwide are utilizing Tandem Control-IQ technology, with 452,000 individuals reported to be using Tandem insulin pumps, two-thirds of whom are on Automated Insulin Delivery (AID).
- Notably, a real-world study involving nearly 20,000 individuals demonstrated a mean Time In Range of 70.5% and Time Below Range of 1.5%.
Updates to Tandem Control-IQ Technology
- Depending on geographical location, Tandem t:slim X2 pump software updates now include integration with Dexcom G7 and FreeStyle Libre 2 Plus. The integration of FreeStyle Libre 2 Plus is currently limited to the US, and it's unclear when or if this will come to Europe. It's possible that in Europe, integration with FreeStyle Libre 3 will be offered directly.
- Additionally, the highly anticipated Tandem Mobi pump, a semi-patch pump, is currently being introduced in the US and is expected to launch in Europe by early 2025. The Control-IQ algorithm is housed within the Mobi pump, which is controlled via the Tandem Mobi Mobile app. The Tandem Mobi currently supports the Dexcom G6 sensor, with plans to introduce integration with the Dexcom G7 sensor in the second quarter of 2024. Subsequently, efforts will be directed towards integrating the FreeStyle Libre 3 sensor.
- Future hardware updates are expected to include the Tandem t:slim X3, Mobi tubeless version, and Sigi pump, although timelines for these releases are uncertain.
- An upgrade from Control-IQ 1.0 to 1.5 was planned for 2024, but updated timelines have not been disclosed. Notably, this update will expand the range of total daily insulin dose (from 10-100 U/d to 5-200 U/d) and weight parameters, allowing for broader user accessibility.
Tandem Control-IQ in Special Populations
- Case studies have demonstrated positive outcomes of Tandem Control-IQ technology in pregnancy, with results from the CIRCUIT study anticipated in 2026.
- Furthermore, Tandem Control-IQ has recently received approval in the US for children aged 2-6 years, with approval pending in Europe.
- Encouraging real-world data from individuals with type 2 diabetes using Tandem Control-IQ technology was presented at ATTD2023. An ongoing pivotal trial is nearing completion, with CEO Mr. Sheridan anticipating market availability for individuals with type 2 diabetes by 2025. Additionally, results from the Close AP+ Study showcased notable improvements in Time In Range when utilizing Tandem Control-IQ in individuals with type 2 diabetes requiring home nursing assistance.
Tandem Control-IQ with Newer Insulins
- Tandem Control-IQ is approved for use with Novorapid, Novolog, or Humalog 100 U/ml insulins.
- At ATTD2024, a prospective study demonstrated positive patient satisfaction with Control-IQ 1.5 and Lyumjev, despite minimal glycemic improvements.
Advancements Towards a Full AID System
- Research presented at ATTD2024 explored the impact of not administering mealtime boluses for a week among Tandem Control-IQ users. Tandem analyzed data from January 2020 to mid-2023, finding that 9% of users didn't bolus for at least seven days. Despite this, they achieved a median Time in Range (TIR) of 62% with Control-IQ as a fully closed-loop (FCL) AID system, slightly higher than the 59% TIR with boluses in hybrid closed loop mode (HCL). It's unclear why some users go without bolusing for extended periods and why TIR is higher in FCL compared to HCL (62% vs. 59%).
3. Omnipod 5
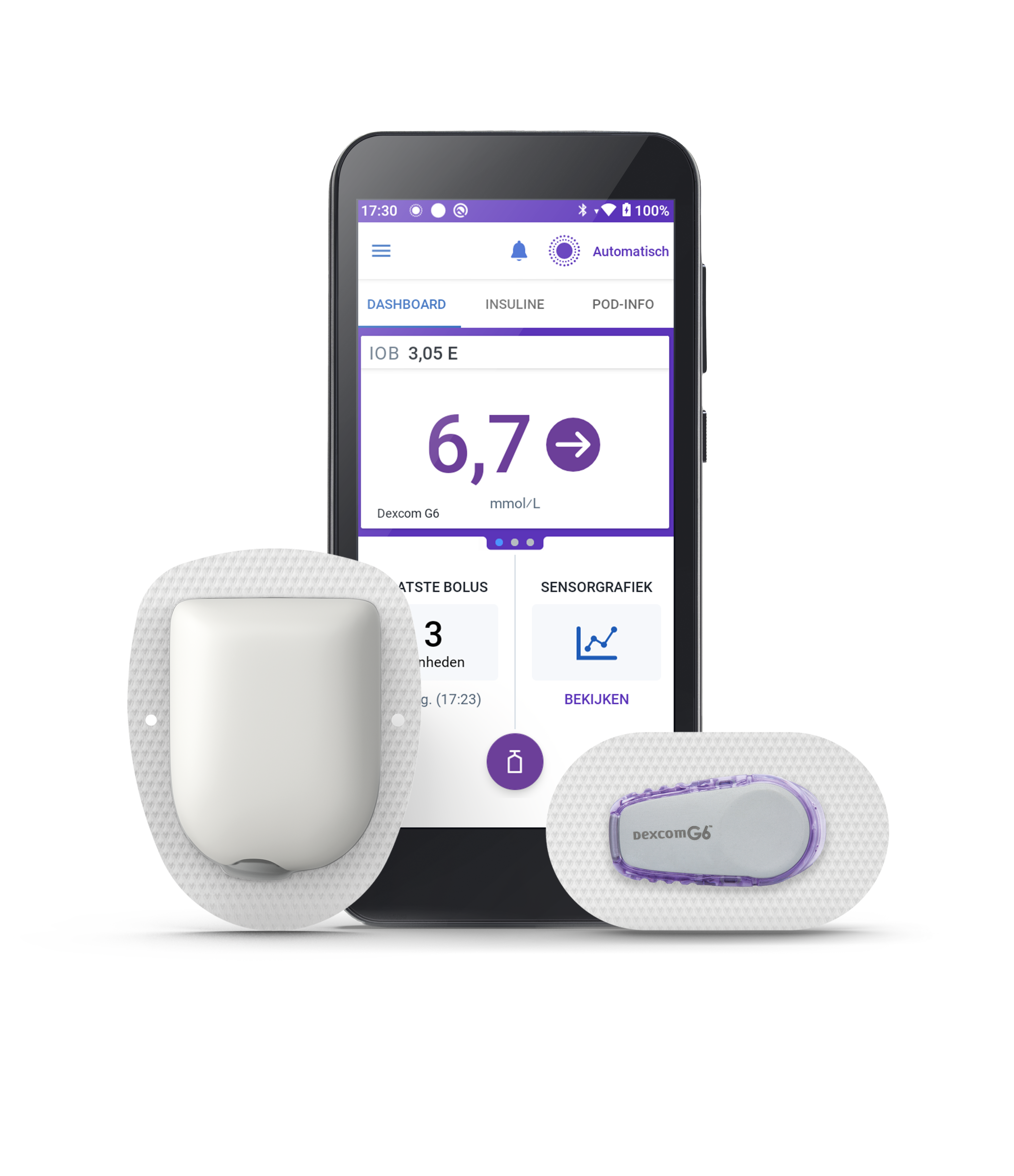
Omnipod 5 Usage and Real-World Performance
-
The number of Omnipod 5 users worldwide is estimated to be around 250,000. While it has been available in the US for two years, its rollout in Europe has been slow, with initial availability limited to individuals with type 1 diabetes in Germany and the UK. Commercialization efforts are ongoing, with plans to launch in the Netherlands soon and expand across Europe by the end of 2024. Insulet mentioned Denmark, Finland, Italy, Norway and Sweden as targets for future launches.
-
Real-world data from nearly 70,000 users demonstrated a mean Time In Range of 68.8% and Time Below Range of 1.1%. Similar results were observed in the first European users, with a mean Time In Range of 68% among users in the UK and Germany who used the most agressive target of 110 mg/dl or 6.1 mmol/l. Additionally, a randomized controlled trial in Europe showed an increase in Time In Range from 44% to 61% among adults with type 1 diabetes.
Omnipod 5 Product Updates
-
Omnipod 5 works with the Dexcom G6 sensor, but is also approved with Dexcom G7 in the US, and with the FreeStyle Libre 2 Plus sensor in Europe. Limited market releases with these sensors have been initiated, with further launches planned.
-
Omnipod 5 can be controlled through a handset or via the Omnipod 5 app on Android phones in the US, with an iPhone app expected soon. However, availability of the app in Europe remains unclear.
Omnipod 5 in Special Populations
-
While there is currently no data on Omnipod 5 use in pregnancy, we heard that some people are using it off-label with good results.
-
Omnipod 5 is officially approved for children aged 2 and older, with positive glycemic outcomes observed in preschoolers for up to 2 years.
-
Positive real-world data in individuals with type 2 diabetes has also been reported, with results from the SECURE-T2D trial anticipated at ADA2024.
Omnipod 5 with Newer Insulins
-
Omnipod 5 is approved for use with Novorapid, Novolog, Trurapi, Kirsty, Humalog or Admelog 100 U/ml insulins.
-
Although there is currently no available data on this, there have been reports of individuals using concentrated insulin such as Humalog 200 U/ml in the Omnipod 5 system. This is done to accommodate individuals who require more than 200 units of insulin every three days. Additionally, some individuals use a once-daily long-acting insulin alongside the Omnipod 5 system for those requiring over 200 units of insulin per day. It's important to note that these practices constitute off-label use, and there is a lack of data supporting these approaches.
Advancements Towards a Full AID System
-
Exciting developments in the next-gen Omnipod 5 algorithm were presented at ATTD2024. The EVOLUTION trial evaluated Insulet's fully closed-loop algorithm in a group of 9 adults with type 1 diabetes and 8 adults with type 2 over a period of three weeks. In individuals with type 1 diabetes, Time In Range (TIR) surged from 37% to 58%, while for those with type 2 diabetes, TIR rose from 52% to 65%.
4. CamAPS FX
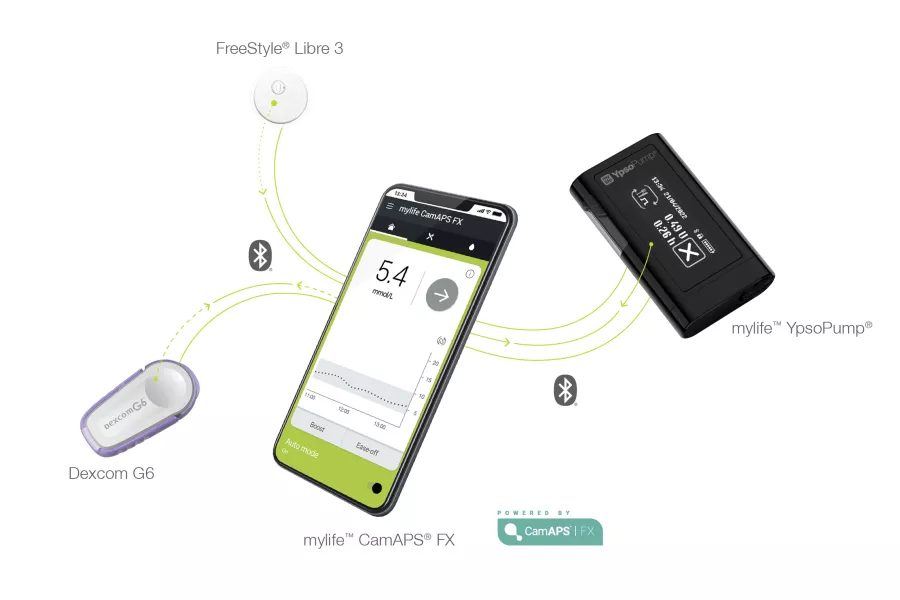
CamAPS FX Usage and Real-World Performance
-
Ypsomed reported in November 2023 that approximately 40,000 individuals were using the mylife Loop system, with likely increased adoption since then, particularly if we include regular CamAPS FX users in the UK.
-
At ATTD2023, real-world data from 1805 users showed a mean Time In Range of 72.6% and Time Below Range of 2.3%. Recent findings indicate that integration with FreeStyle Libre 3 also yields favorable results, with a mean Time In Range of 72.6% and Time Below Range of 3.1% among 100 users.
Product Updates for CamAPS FX
- The mylife Loop system currently integrates with Dexcom G6 sensors, and integration with FreeStyle Libre 3 sensors is being gradually introduced across various European countries. The timeline for integration with Dexcom G7 sensors remains uncertain.
- CEO Mr. Michel shared during the latest investor update that CamDiab filed its mylife CamAPS FX App with the FDA in August 2023, with plans for the mylife YpsoPump to follow suit in the "coming months," aiming for FDA approval by mid FY25.
- While the mylife CamAPS FX app is currently available for Android phones, plans for an iOS app have been discussed, although no recent updates on timelines have been provided.
- Additionally, CamDiab is developing the CamAPS HX algorithm, designed for use without meal boluses, as part of their ongoing efforts.
CamAPS FX in Special Populations
- CamAPS FX is the only officially approved algorithm for use in pregnancy, with results from the AiDAPT Trial being highlighted at EASD2023.
- It is also the approved algorithm for children with diabetes aged 1 and older.
- For people with type 2 diabetes, CamDiab primarily conducts studies with their full AID system, CamAPS HX.
CamAPS FX with Newer Insulins
- CamAPS FX is approved for use with all U100 rapid-acting and ultra-rapid-acting insulin.
- While trials with Fiasp did not reveal significant differences in glucose control, recent studies indicate that using Lyumjev may lead to improved postprandial glucose excursions.
Advancements Towards a Full AID System
- At ATTD2024, results from the SMASH study were presented, indicating that simplified meal announcements are as effective as carb counting. The study involved 46 individuals aged 12-20 with type 1 diabetes who utilized the CamAPS FX system with Dexcom G6 sensors. Participants followed either a simplified meal announcement protocol or precise carbohydrate counting for three months each in random order. Notably, there was no disparity in Time In Range (TIR) (70% vs. 71%) or Time Below Range (TBR) (1.8% vs. 1.9%) between the two approaches. This suggests a potential reduction in mealtime burden for individuals who struggle with carb counting.
- CamDiab is also progressing with their fully closed-loop algorithm, CamAPS HX, with positive findings published in 26 people with type 2 diabetes, 32 people with diabetes in the hospital, and in 26 people with type 1 diabetes. A recent study also emphasises the positive effect on quality-of-life of these fully closed-loop systems in people with type 1 diabetes.
5. Diabeloop

Diabeloop Usage and Real-World Performance
-
The exact number of Diabeloop users is not disclosed by the company, but it was previously mentioned that more than 10,000 individuals were using it.
-
A real-world study involving 3,706 users demonstrated an average Time In Range (TIR) of 72.1% and Time Below Range (TBR) of 0.9%. Similarly, at ATTD2023, real-world data from 6,658 users showed a mean TIR of 71% and TBR of 0.8%.
Product Updates for Diabeloop
- Since the introduction of Diabeloop-Kaleido, significant updates have been made to the handset, including a shift to a white design from the previous black, along with a reduction in size and weight.
- Diabeloop is compatible with four insulin pumps: the Accu-Chek Insight from Roche (now discontinued), the Kaleido insulin pump from ViCentra (currently available in the Netherlands, France, and Germany, with plans for release in the UK and Italy in 2024), the Dana-i insulin pump from SOOIL, and the Medisafe With insulin pump from Terumo (also currently in study).
- Diabeloop is currently compatible with the Dexcom G6 and is expected to integrate with the Dexcom G7 in the future, although a timeline for this update is not yet available.
- An app for Diabeloop is anticipated to be released in 2024, as indicated by Erik Huneker during a Zoom Expert Meeting in January 2024.
- Additionally, Diabeloop is working on integrating exercise parameters from smartwatches into their algorithm, as discussed at EASD2023.
- Diabeloop has developeda separate CE-labeled algorithm for individuals with highly unstable type 1 diabetes (DBLHU), as well as slightly adapted algorithms for children (DBL4K) and teenagers (DBL4T). A study is currently underway in Belgium, France, and Germany to evaluate Diabeloop's performance in adolescents aged 12-18, with results expected in the summer of 2024. This study also includes testing an "Unannounced Meal Management (UMM) module." One goal is to implement this UMM module in the next generation of DBL products.
- Despite no specific timeline available, an FDA and Japanese authorities submission have been discussed.
Diabeloop in Special Populations
- There is currently no data available regarding the use of Diabeloop in pregnancy or preschool-aged children.
- A study with Diabeloop has just been competed in adults with type 2 diabetes in France, and showed a TIR of 76% with a 15 points improvement versus controls. Data are currently considered for publication.
Diabeloop with Newer Insulins
- Diabeloop is approved for use with Novorapid and Humalog 100 U/ml.
- A study with a virtual simulator showed that Diabeloop was safe to use with Lyumjev and Fiasp. When the DBLG1 algorithm was tuned to the specific insulin type, Lyumjev led to a better TIR compared to Novorapid.
Advancements Towards a Full AID System
- Diabeloop features a simplified meal announcement function within their DBLG handset. A recent study demonstrated similar TIR between individuals who used this feature and those who counted carbs.
- The ongoing DBL4T study is evaluating a specific Diabeloop algorithm without meal bolusing, with promising initial results. Further details and plans for the commercialization of this algorithm update are expected by 2025.
6. Open-source AID systems (off-label!)

Open-source AID Usage and Real-World Performance
-
The exact number of individuals using open-source AID systems is currently unknown. However, it's estimated that approximately 10,000 people are utilizing AndroidAPS, 10,0000 people are using open-source Loop (DIY Loop), and around 1,000 people are using iAPS (based on the members in the dedicated Discord and FaceBook groups).
-
A recent real-world study on Supported Open-Source Artificial Pancreas Systems (SOSAPS) involving 248 individuals with type 1 diabetes demonstrated an average Time In Range (TIR) of 80% and Time Below Range (TBR) of 2.5%. The Diabetes Canada position paper also summarizes previous real-world data and the findings of a randomized controlled trial conducted in 2022. Moreover, certain users of iAPS have consented to share their data, allowing you to track their real-time results through this link.
Product Updates for Open-Source AID Systems
- AndroidAPS: Version 3.2.0.0 was released on October 23, 2023, introducing the ability to link the Medtrum Nano insulin pump, as well as compatibility with the Dexcom G7 and Glunovo CGM.
- Open-source Loop (DIY Loop): Version 3.2.3, released on September 19, 2023, now supports linking with the Omnipod DASH and the Dexcom G7 since January 2023.
- iAPS: Version 3.4.0, released on March 18, 2024, is available, although many users recommend sticking with version 2.3.3 from January 9, 2024, due to its extensive testing.
Open-Source AID in Special Populations
- Open-source AID systems have been utilized by individuals during pregnancy, with limited literature available on this topic.
- Some parents use open-source AID systems in children under 6 years old, although specific data regarding this population are lacking.
- Several individuals with type 2 diabetes have adopted open-source AID systems. A recent report involving seven individuals using open-source Loop showed a mean TIR of 93%.
Open-Source AID with Newer Insulins
- Many users of open-source AID systems opt for ultra-fast-acting insulins like Fiasp or Lyumjev.
- Additionally, some individuals may mix their insulins, although there is limited data available on this practice.
Advancements Towards a Full AID System
- Many users utilize open-source AID systems as full AID systems, often activating the Unannounced Meal function. This feature is reported to work effectively with ultra-rapid insulins like Lyumjev.
- Results from the Pancreas4All study, presented at ISPAD2023, demonstrated the feasibility of using AndroidAPS as a full closed-loop system. This study involved 16 individuals with type 1 diabetes using AndroidAPS with carb counting, simple meal announcement, and without meal boluses, each for three days. No clinically significant differences in glucose control were observed, indicating the potential efficacy of AndroidAPS as a full closed-loop system.
7. Insulin-only iLet BetaBionics
iLet Usage and Real-World Performance
-
The iLet system began commercialization in the US in the summer of 2023, but specific user numbers remain undisclosed.
-
Although real-world data is not yet available, a pivotal trial involving 219 individuals with type 1 diabetes revealed an average Time in Range (TIR) of 65% and Time Below Range (TBR) of 1.8%.
iLet Product Updates
- The iLet Bionic Pancreas received FDA approval on May 22, 2023, for individuals with type 1 diabetes aged 6 years and older. However, it's unclear if a CE-label has been sought, and rollout plans beyond the US have not been disclosed.
- The iLet Device is compatible with Dexcom G6 and Dexcom G7 sensors. Beta Bionics has established a partnership with Sensionics for potential integration with the Eversense sensor, though no timeline has been provided.
- Additionally, Beta Bionics is developing a dual hormone AID system called iLet Duo, which contains insulin and glucagon. Initial results from 20 adults with type 1 diabetes showed promising outcomes, with a TIR of 78.4%. However, a larger study on iLet Duo, initially slated for 2023, has been delayed multiple times.
iLet in Special Populations
- There is currently no available data on the use of the iLet system in pregnancy or in preschool-aged children.
- Dr. Steven Russell, Chief Medical Officer at Beta Bionics, mentioned in an interview that preliminary studies suggest the insulin-only version of the iLet may be beneficial for individuals with type 2 diabetes. However, these studies are unpublished and in the early stages.
iLet with Newer Insulins
- The iLet system is approved for use with U100 Novorapid, Humalog, and Fiasp PumpCart insulin formulations.
Towards a Full AID System
- Users of the iLet Device rely on meal announcements rather than carb counting. While there are no current indications that Beta Bionics is developing a full AID system, ongoing developments may follow as they assess the outcomes of meal announcement-based approaches.
8. Emerging AID Systems
Here are some new AID systems currently emerging:
1. AP6 (INREDA)

- This AID system utilizes dual hormones, insulin, and glucagon, and has obtained a CE-label for adults with type 1 diabetes. A phase 3 trial involving 240 adults with type 1 diabetes is currently underway in the Netherlands, along with a study involving the use of Lyumjev. Recent research demonstrated promising outcomes in 70 adults with type 1 diabetes who used the AP5 system for one year, with a mean Time in Range (TIR) of 80.3% and Time Below Range (TBR) of 1.3%.
2. TouchCare Nano System (Medtrum)

- This AID system is reportedly available for commercial use in several countries, including the Czech Republic (with 150 users), Estonia (with 70 users), Greece (with 30 users), and Slovenia (with 10 users), as well as in Sweden, Denmark, the UK, Italy, Hungary, and Slovenia (information provided by a Medtrum representative at the ATTD2024 booth). However, scientific information or manuals for this system are not readily available online.
3. Tidepool Loop (Tidepool)

- FDA clearance for this AID system was obtained in January 2023 for individuals with type 1 diabetes aged 6 years and older. It is based on an older version of the open-source Loop algorithm and is designed for use with an iPhone, although development of an Android app is underway. The Tidepool Loop system will be compatible with Dexcom sensors, and will be launched soon in the US with the twiist pump from Sequel Med Tech. There are also ongoing studies with the Embecta pump for potential FDA clearance for use in individuals with type 2 diabetes.
9. Diabetes Simulators for Accelerated Results
Traditionally, we have heavily relied on human testing for every new diabetes algorithm, a process that consumes significant time and resources.
However, there is a growing movement advocating for the adoption of modern techniques like virtual simulators to test AID algorithms.
This shift holds promise for significantly expediting progress in this field.
Unfortunately, there is currently a scarcity of official simulators available.
However, at ATTD2024, prof Peuscher (Ulm University of Applied Sciences, Germany) introduced several open-source diabetes simulators developed by the community (and himself).
These tools, though not exhaustive, are listed below to ensure researchers are aware of these free resources,
which can expedite their investigations and potentially provide quicker access to enhanced AID algorithms.
- Diabetes Type 1 Simulators: Simglucose, LoopInsighT1 (LT1), Closed-Loop Testbed for Resiliende Design and Assessment of APS, Tidepool Simulator
- Diabetes Type 2 Simulators: CarbMetSim, T2DSim, A Type 2 diabetes simulator with web based GUI
- Real-time Simulator: CGMSim
- Personalised Simulation / Digital Twin: ReplayBG, Simulator for T1D with aerobic exercise, Sirael
- Tools: API for the UVA/Padova Type 1 Diabetes Metabolic Simulator, GluPredKit
- Data Analysis: AGATA (Automated Glucose dATa Analysis)
Given the potential for exponential improvement in AID algorithms, especially with the integration of artificial intelligence and additional parameters such as exercise, heart rate, ketones, and lactate, researchers are encouraged to explore these free resources when conducting AID algorithm research.
There is a vibrant community actively working on these open-source diabetes simulators.
If you're interested to learn more about these open-source solutions, feel free to reach out to the authors and join their Discord chat here or participate in their online meetings.
10. AID Systems to be Cost-Effective for People with Type 1 Diabetes

As AID systems are becoming the standard of care for those with type 1 diabetes, similar glycemic outcomes are now being observed in individuals with type 2 diabetes.
However, the question remains: will these systems be covered by insurance?
This hinges on lobbying efforts and the willingness of each country to allocate funds.
A publication by Prof Chantal Mathieu and Pieter Gillard describes that although the evidence is still limited, most data indicate that AID systems cost less than the willingness to pay per QALY gained across different countries.
There also give recommendations to ensure that all the benefits of AID systems are thoroughly considered in these analyses.
The hope is that this evidence will establish the cost-effectiveness of AID systems for individuals with all forms of insulin-dependent diabetes, including those with type 2 diabetes who require insulin therapy.
In summary, significant advancements are underway for the available AID systems, with numerous updates on the horizon.
Furthermore, we anticipate the introduction of several new AID systems to the market, which we embrace as greater options can translate to enhanced care.
If you're interested in familiarizing yourself with all AID systems and accessing comprehensive educational resources in one convenient location, look no further!
We've developed time-efficient videos covering all AID systems, accessible from your phone and offered in 30 languages.
Kind regards,